I'd like to introduce you to an emerging area of science,
one that is still speculative but hugely exciting, and certainly one that's
growing very rapidly. Quantum biology asks a very simple question: Does quantum
mechanics -- that weird and wonderful and powerful theory of the subatomic
world of atoms and molecules that underpins so much of modern physics and
chemistry -- also play a role inside the living cell? In other words: Are there
processes, mechanisms, phenomena in living organisms that can only be explained
with a helping hand from quantum mechanics? Now, quantum biology isn't new;
it's been around since the early 1930s. But it's only in the last decade or so
that careful experiments -- in biochemistry labs, using spectroscopy -- have
shown very clear, firm evidence that there are certain specific mechanisms that
require quantum mechanics to explain them.
01:17
Quantum biology brings together quantum physicists,
biochemists, molecular biologists -- it's a very interdisciplinary field. I
come from quantum physics, so I'm a nuclear physicist.
01:28
I've spent more than three decades trying to get my head
around quantum mechanics. One of the founders of quantum mechanics, Niels Bohr,
said, If you're not astonished by it, then you haven't understood it. So I sort
of feel happy that I'm still astonished by it. That's a good thing. But it
means I study the very smallest structures in the universe -- the building
blocks of reality. If we think about the scale of size, start with an everyday
object like the tennis ball, and just go down orders of magnitude in size --
from the eye of a needle down to a cell, down to a bacterium, down to an enzyme
-- you eventually reach the nano-world.
02:09
Now, nanotechnology may be a term you've heard of. A
nanometer is a billionth of a meter. My area is the atomic nucleus, which is
the tiny dot inside an atom. It's even smaller in scale. This is the domain of
quantum mechanics, and physicists and chemists have had a long time to try and
get used to it. Biologists, on the other hand, have got off lightly, in my
view. They are very happy with their balls-and-sticks models of molecules.
02:38
(Laughter)
02:39
The balls are the atoms, the sticks are the bonds between
the atoms. And when they can't build them physically in the lab, nowadays, they
have very powerful computers that will simulate a huge molecule. This is a
protein made up of 100,000 atoms. It doesn't really require much in the way of
quantum mechanics to explain it. Quantum mechanics was developed in the 1920s.
It is a set of beautiful and powerful mathematical rules and ideas that explain
the world of the very small. And it's a world that's very different from our
everyday world, made up of trillions of atoms. It's a world built on
probability and chance. It's a fuzzy world. It's a world of phantoms, where
particles can also behave like spread-out waves.
03:30
If we imagine quantum mechanics or quantum physics, then, as
the fundamental foundation of reality itself, then it's not surprising that we
say quantum physics underpins organic chemistry. After all, it gives us the
rules that tell us how the atoms fit together to make organic molecules.
Organic chemistry, scaled up in complexity, gives us molecular biology, which
of course leads to life itself. So in a way, it's sort of not surprising. It's
almost trivial. You say, "Well, of course life ultimately must depend of
quantum mechanics." But so does everything else. So does all inanimate
matter, made up of trillions of atoms.
04:08
Ultimately, there's a quantum level where we have to delve
into this weirdness. But in everyday life, we can forget about it. Because once
you put together trillions of atoms, that quantum weirdness just dissolves
away. Quantum biology isn't about this. Quantum biology isn't this obvious. Of
course quantum mechanics underpins life at some molecular level. Quantum
biology is about looking for the non-trivial -- the counterintuitive ideas in
quantum mechanics -- and to see if they do, indeed, play an important role in
describing the processes of life.
04:54
Here is my perfect example of the counter-intuitiveness of
the quantum world. This is the quantum skier. He seems to be intact, he seems
to be perfectly healthy, and yet, he seems to have gone around both sides of
that tree at the same time. Well, if you saw tracks like that you'd guess it
was some sort of stunt, of course. But in the quantum world, this happens all
the time. Particles can multitask, they can be in two places at once. They can
do more than one thing at the same time. Particles can behave like spread-out
waves. It's almost like magic.
05:27
Physicists and chemists have had nearly a century of trying
to get used to this weirdness. I don't blame the biologists for not having to
or wanting to learn quantum mechanics.
05:37
You see, this weirdness is very delicate; and we physicists
work very hard to maintain it in our labs. We cool our system down to near
absolute zero, we carry out our experiments in vacuums, we try and isolate it
from any external disturbance. That's very different from the warm, messy,
noisy environment of a living cell. Biology itself, if you think of molecular
biology, seems to have done very well in describing all the processes of life
in terms of chemistry -- chemical reactions. And these are reductionist,
deterministic chemical reactions, showing that, essentially, life is made of
the same stuff as everything else, and if we can forget about quantum mechanics
in the macro world, then we should be able to forget about it in biology, as
well.
06:27
Well, one man begged to differ with this idea. Erwin
Schrödinger, of Schrödinger's Cat fame, was an Austrian physicist. He was one
of the founders of quantum mechanics in the 1920s. In 1944, he wrote a book
called "What is Life?" It was tremendously influential. It influenced
Francis Crick and James Watson, the discoverers of the double-helix structure
of DNA. To paraphrase a description in the book, he says: At the molecular
level, living organisms have a certain order, a structure to them that's very
different from the random thermodynamic jostling of atoms and molecules in
inanimate matter of the same complexity.
07:13
In fact, living matter seems to behave in this order, in a
structure, just like inanimate matter cooled down to near absolute zero, where
quantum effects play a very important role. There's something special about the
structure -- the order -- inside a living cell. So, Schrödinger speculated that
maybe quantum mechanics plays a role in life. It's a very speculative,
far-reaching idea, and it didn't really go very far.
07:45
But as I mentioned at the start, in the last 10 years, there
have been experiments emerging, showing where some of these certain phenomena
in biology do seem to require quantum mechanics.
07:55
I want to share with you just a few of the exciting ones.
This is one of the best-known phenomena in the quantum world, quantum
tunneling.
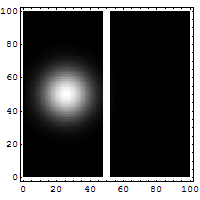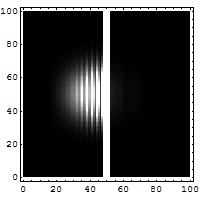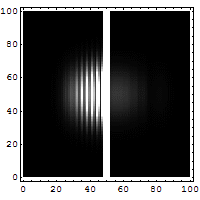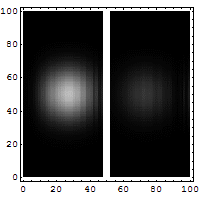
The box on the left shows the wavelike, spread-out distribution of a
quantum entity -- a particle, like an electron, which is not a little ball
bouncing off a wall. It's a wave that has a certain probability of being able
to permeate through a solid wall, like a phantom leaping through to the other
side. You can see a faint smudge of light in the right-hand box. Quantum
tunneling suggests that a particle can hit an impenetrable barrier, and yet
somehow, as though by magic, disappear from one side and reappear on the other.
The nicest way of explaining it is if you want to throw a ball over a wall, you
have to give it enough energy to get over the top of the wall. In the quantum
world, you don't have to throw it over the wall, you can throw it at the wall,
and there's a certain non-zero probability that it'll disappear on your side,
and reappear on the other.
08:57
This isn't speculation, by the way. We're happy -- well,
"happy" is not the right word --
09:02
(Laughter)
09:04
we are familiar with this.
09:06
(Laughter)
09:09
Quantum tunneling takes place all the time; in fact, it's
the reason our Sun shines. The particles fuse together, and the Sun turns
hydrogen into helium through quantum tunneling. Back in the 70s and 80s, it was
discovered that quantum tunneling also takes place inside living cells.
Enzymes, those workhorses of life, the catalysts of chemical reactions --
enzymes are biomolecules that speed up chemical reactions in living cells, by
many, many orders of magnitude. And it's always been a mystery how they do
this.
09:43
Well, it was discovered that one of the tricks that enzymes
have evolved to make use of, is by transferring subatomic particles, like
electrons and indeed protons, from one part of a molecule to another via
quantum tunneling. It's efficient, it's fast, it can disappear -- a proton can
disappear from one place, and reappear on the other. Enzymes help this take
place.
10:08
This is research that's been carried out back in the 80s,
particularly by a group in Berkeley, Judith Klinman. Other groups in the UK
have now also confirmed that enzymes really do this.
10:21
Research carried out by my group -- so as I mentioned, I'm a
nuclear physicist, but I've realized I've got these tools of using quantum
mechanics in atomic nuclei, and so can apply those tools in other areas as
well. One question we asked is whether quantum tunneling plays a role in
mutations in DNA. Again, this is not a new idea; it goes all the way back to
the early 60s. The two strands of DNA, the double-helix structure, are held
together by rungs; it's like a twisted ladder. And those rungs of the ladder
are hydrogen bonds -- protons, that act as the glue between the two strands. So
if you zoom in, what they're doing is holding these large molecules --
nucleotides -- together. Zoom in a bit more. So, this a computer simulation.
The two white balls in the middle are protons, and you can see that it's a
double hydrogen bond. One prefers to sit on one side; the other, on the other side
of the two strands of the vertical lines going down, which you can't see. It
can happen that these two protons can hop over. Watch the two white balls. They
can jump over to the other side. If the two strands of DNA then separate,
leading to the process of replication, and the two protons are in the wrong
positions, this can lead to a mutation.
11:43
This has been known for half a century. The question is: How
likely are they to do that, and if they do, how do they do it? Do they jump
across, like the ball going over the wall? Or can they quantum-tunnel across,
even if they don't have enough energy? Early indications suggest that quantum
tunneling can play a role here. We still don't know yet how important it is;
this is still an open question. It's speculative, but it's one of those
questions that is so important that if quantum mechanics plays a role in
mutations, surely this must have big implications, to understand certain types
of mutations, possibly even those that lead to turning a cell cancerous.
12:22
Another example of quantum mechanics in biology is quantum
coherence, in one of the most important processes in biology, photosynthesis:
plants and bacteria taking sunlight, and using that energy to create biomass.
Quantum coherence is the idea of quantum entities multitasking. It's the
quantum skier. It's an object that behaves like a wave, so that it doesn't just
move in one direction or the other, but can follow multiple pathways at the
same time.
12:54
Some years ago, the world of science was shocked when a
paper was published showing experimental evidence that quantum coherence takes
place inside bacteria, carrying out photosynthesis. The idea is that the
photon, the particle of light, the sunlight, the quantum of light captured by a
chlorophyll molecule, is then delivered to what's called the reaction center,
where it can be turned into chemical energy. And in getting there, it doesn't
just follow one route; it follows multiple pathways at once, to optimize the
most efficient way of reaching the reaction center without dissipating as waste
heat. Quantum coherence taking place inside a living cell. A remarkable idea,
and yet evidence is growing almost weekly, with new papers coming out,
confirming that this does indeed take place.
13:45
My third and final example is the most beautiful, wonderful
idea. It's also still very speculative, but I have to share it with you. The
European robin migrates from Scandinavia down to the Mediterranean, every
autumn, and like a lot of other marine animals and even insects, they navigate
by sensing the Earth's magnetic field. Now, the Earth's magnetic field is very,
very weak; it's 100 times weaker than a fridge magnet, and yet it affects the
chemistry -- somehow -- within a living organism. That's not in doubt -- a
German couple of ornithologists, Wolfgang and Roswitha Wiltschko, in the 1970s,
confirmed that indeed, the robin does find its way by somehow sensing the
Earth's magnetic field, to give it directional information -- a built-in
compass.
14:37
The puzzle, the mystery was: How does it do it? Well, the
only theory in town -- we don't know if it's the correct theory, but the only
theory in town -- is that it does it via something called quantum entanglement.
Inside the robin's retina -- I kid you not -- inside the robin's retina is a
protein called cryptochrome, which is light-sensitive. Within cryptochrome, a
pair of electrons are quantum-entangled. Now, quantum entanglement is when two
particles are far apart, and yet somehow remain in contact with each other.
Even Einstein hated this idea; he called it "spooky action at a
distance."
15:12
(Laughter)
15:14
So if Einstein doesn't like it, then we can all be
uncomfortable with it. Two quantum-entangled electrons within a single molecule
dance a delicate dance that is very sensitive to the direction the bird flies
in the Earth's magnetic field.
15:26
We don't know if it's the correct explanation, but wow,
wouldn't it be exciting if quantum mechanics helps birds navigate? Quantum
biology is still in it infancy. It's still speculative. But I believe it's
built on solid science. I also think that in the coming decade or so, we're
going to start to see that actually, it pervades life -- that life has evolved
tricks that utilize the quantum world. Watch this space.
16:01
Thank you.
16:02